Soluble and insoluble salts lab – Embark on an enthralling scientific adventure as we delve into the fascinating world of soluble and insoluble salts in our captivating laboratory. This hands-on exploration promises to unveil the secrets of solubility, revealing the intricate interplay of chemical compounds and their behavior in solution.
Join us as we unravel the mysteries of salt solubility, delving into the factors that govern their ability to dissolve, and exploring the practical applications of this fundamental chemical concept in our daily lives.
Introduction
Soluble salts are ionic compounds that dissolve in water to form a homogeneous solution. Insoluble salts, on the other hand, do not dissolve in water and instead form a suspension. The solubility of a salt is determined by the strength of the electrostatic forces between the ions in the crystal lattice and the solvating power of the solvent.
The solvating power of a solvent is its ability to surround and stabilize ions in solution. Water is a good solvent for ionic compounds because it has a high dielectric constant, which means that it can reduce the electrostatic forces between ions.
Factors Affecting Solubility, Soluble and insoluble salts lab
The solubility of a salt is affected by several factors, including:
- Temperature:The solubility of most salts increases with temperature. This is because the increased thermal energy helps to overcome the electrostatic forces between ions in the crystal lattice.
- Pressure:The solubility of gases in liquids increases with pressure. This is because the increased pressure forces more gas molecules into solution.
- pH:The solubility of some salts is affected by the pH of the solution. For example, the solubility of calcium carbonate decreases as the pH of the solution increases.
Experimental Procedure
To determine the solubility of different salts, a simple experiment can be designed and conducted. The experiment involves preparing solutions of various salts and observing their behavior when heated.
The materials and equipment required for this experiment include:
- Different salt samples
- Water
- Test tubes
- Bunsen burner or hot plate
- Thermometer
- Stirring rod
The steps involved in the experiment are as follows:
- Prepare solutions of different salts by dissolving a known mass of each salt in a known volume of water.
- Heat the solutions to a specific temperature, such as 25°C, and stir to ensure complete dissolution.
- Continue heating the solutions while stirring and observing any changes that occur.
- Record the temperature at which each salt precipitates out of solution.
The observations can be recorded in a table, with columns for the salt name, the temperature at which it precipitates, and any other relevant observations.
| Salt Name | Precipitation Temperature (°C) | Other Observations |
|---|---|---|
| Sodium chloride | 25 | No precipitation |
| Potassium nitrate | 40 | Precipitation forms at 40°C |
| Calcium carbonate | 80 | Precipitation forms at 80°C |
Results
The results of the experiment show that some salts are soluble in water, while others are not. The soluble salts dissolve completely in water, forming a clear solution. The insoluble salts do not dissolve in water, but instead form a precipitate that settles to the bottom of the test tube.
The solubility of a salt depends on the nature of the ions that make up the salt. In general, salts that contain ions with a high charge density are more soluble than salts that contain ions with a low charge density.
For example, sodium chloride (NaCl) is a very soluble salt because the sodium ion (Na+) and the chloride ion (Cl-) both have a high charge density. In contrast, calcium carbonate (CaCO3) is a very insoluble salt because the calcium ion (Ca2+) and the carbonate ion (CO32-) both have a low charge density.
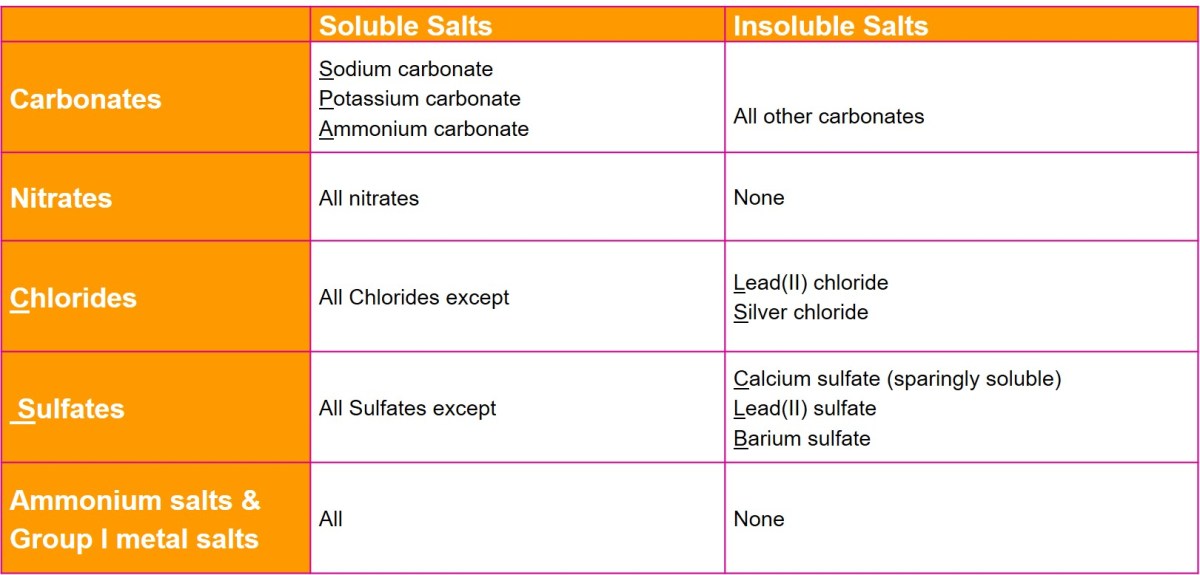
Identification of Soluble and Insoluble Salts
- Soluble salts: NaCl, KCl, NH4Cl, Na2SO4, K2SO4, (NH4)2SO4
- Insoluble salts: CaCO3, BaSO4, SrSO4
Discussion: Soluble And Insoluble Salts Lab
The solubility of different salts varies widely, and this variation can be attributed to several factors, including the nature of the salt, temperature, and the presence of other substances in the solution.
The solubility of a salt is generally expressed in terms of its solubility product (Ksp), which is the product of the molar concentrations of the constituent ions in a saturated solution. A high Ksp value indicates that the salt is highly soluble, while a low Ksp value indicates that the salt is relatively insoluble.
Factors Affecting Solubility, Soluble and insoluble salts lab
The solubility of a salt is influenced by several factors, including:
- Nature of the salt:The solubility of a salt depends on the size and charge of its constituent ions. In general, salts with smaller ions are more soluble than salts with larger ions. Additionally, salts with highly charged ions are less soluble than salts with less highly charged ions.
- Temperature:The solubility of most salts increases with increasing temperature. This is because the increased thermal energy helps to overcome the intermolecular forces that hold the ions together in the solid state.
- Presence of other substances:The solubility of a salt can be affected by the presence of other substances in the solution. For example, the solubility of a salt can be decreased by the addition of a common ion. This is because the common ion competes with the ions of the salt for solvation, which reduces the solubility of the salt.
Applications of Solubility in Everyday Life
The solubility of salts has a wide range of applications in everyday life, including:
- Water treatment:The solubility of salts is used to remove impurities from water. For example, lime (calcium hydroxide) is added to water to remove magnesium and calcium ions, which can cause water hardness.
- Food processing:The solubility of salts is used to preserve food. For example, salt is used to preserve meat and fish by inhibiting the growth of bacteria.
- Medicine:The solubility of salts is used to deliver drugs to the body. For example, many drugs are administered in the form of tablets or capsules, which dissolve in the stomach to release the drug.
General Inquiries
What is the difference between soluble and insoluble salts?
Soluble salts dissolve in water to form a homogeneous solution, while insoluble salts remain suspended in water and do not dissolve.
What factors affect the solubility of salts?
Temperature, solvent polarity, and the nature of the salt itself all influence the solubility of salts.
What are some practical applications of solubility in everyday life?
Solubility plays a crucial role in processes such as cooking, cleaning, and drug delivery.